In this second article of our remote research series, we’re focusing on operational aspects of the VERKKO study and remote studies in general. In case you missed our first article about the role of the site, you can still access it here.
Conducting a virtual study is an innovative approach requiring the clinical research team to learn some new skills and handle tasks differently. For example, instead of investing time explaining the basics of a study to patients and training them, they now need to guide study candidates in technology-supported processes.
The registered patient population in VERKKO had an average age of 59. Several individuals were over 70 years of age. Some participants were not intimately familiar with everyday technologies, such as email, and needed minimal guidance in activating their Clinpal accounts. In general, participants required more support early on in the process, and once they had completed their electronic informed consent they were able to rely on the instructions in Clinpal. Call centers and help desks can also be established to support study candidates that are new to common technologies, such as email and mobile messaging.
Below is an example of a question from a patient that can be easily answered by a call center or the site.
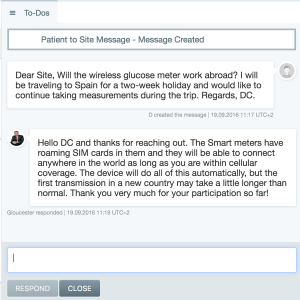 Clinpal Messaging – Direct support to participants
Clinpal Messaging – Direct support to participants

Terminology is important – especially in written form
One benefit of working in a clinical research technology platform, such as Clinpal, is the project team’s access to detailed throughput metrics tracking the progress of the patient workflows. This, combined with input from the site, helps identify bottlenecks in the process and issues that might be confusing to study participants. For example, in VERKKO, the account activation emails used the default ‘validity period’ of 24 hours, after which they automatically expire. The system handles expired activation links graciously and informs the user that their link is expired and will automatically send a new one to their email. Activating online accounts has become a fairly common activity for the general public. Having visibility into the throughput metrics, offered through Clinpal, we could easily and quickly see when study candidates on the VERKKO study had delays or challenges in activating their accounts within the 24-hour timeframe. Looking at the data provided through the Clinpal platform and by reviewing participant feedback, we were quickly alerted to a translation issue that was creating a bottleneck. In Finnish, the message was along the lines of “Your key is expired. We will send you a new one.” This was a not a very well worded translation and some study candidates were actually expecting us to send them a key in the mail.
Aided by the analytics tools in Clinpal, we quickly understood the situation and improved the instructions by advising study candidates to look for a “new message in their email inbox.” Our Clinpal metrics and the site feedback confirmed that the process improved and candidates were more self-reliant during this part of the process.
In VERKKO, participants were required to use a wireless glucose meter that was supplied directly to them and to follow a structured profiling protocol that required a minimum of 25 readings to complete the profile. Once patients started their glucose profiling process, they needed to follow very specific time windows and conduct enough measurements for overnight fast, breakfast and their main meal of the day, based on their personal daily routine. In order to complete the task, they needed to understand and follow the study protocol closely. It was remarkable how well patients were able to do this just based on their remote self-education in Clinpal. In the end, the study demonstrated an 18% increase in compliance, meaning that there were fewer readings done incorrectly and also a 22% improvement in the time it took participants to complete their profiling. These improvement percentages are based on a comparison to a previously conducted study that utilized the same profiling mechanism, but with a traditional non-Clinpal approach.
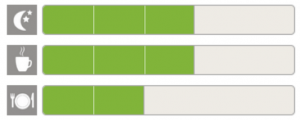 Dashboard report for patients to show their profiling progress
Dashboard report for patients to show their profiling progress
There were at least four key reasons for the increased compliance:
- The remote electronic consenting process was effective in ensuring participants understood what is expected of them;
- The smart glucose meter actively reminded participants to take measurements at the right times;
- The participants had real-time access to their own measurements and a personal dashboard where they could visually see how many more measurements they need;
- The site had access to the same information and was able to intervene remotely when they noticed compliance issues
 Logbook view with a single ‘out-of-bounds’ measurement
Logbook view with a single ‘out-of-bounds’ measurement
The process worked remarkably well from the start. The team kept a close eye on the first participant in the study. We were happy to see that the first study participant only missed one measurement of the 25 required measurements. Through the use of technology-enabled research, we could see that only one of the 25 measurements was conducted 13 minutes outside of the time window. After some gentle direction from the study team and through the use of Clinpal, we could see that on the very next day, the study candidate adhered to the protocol, and completed her measurements nearly perfectly and in the fastest time possible.
The report below shows one participant that was doing measurements routinely, but outside of the time windows corresponding to their personal routines. The site intervened and provided personal guidance, which was effective in getting this participant back on track.
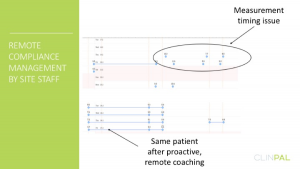 Compliance before and after remote coaching
Compliance before and after remote coaching
In some cases, study participants in a technology-enabled environment, have expectations for an immediate response from the site.
As a result of VERKKO, we learned to manage the expectation of a 24-hour response from the site and as a result, have incorporated a best practice into our future remote trials.
Here is what happened: The study nurse reported that one individual who had just completed her online pre-screener, called the site only 15 minutes later and was frustrated because her application to join the study had not yet been processed. She literally expected that someone would be at the other end doing that in nearly real time. The nurse patiently explained to her that she had finished for the day already, but would review the application once she got to her home computer that evening. Once she did get to the computer, she had already received a message from the candidate who was disappointed that still nobody had processed her request. In retrospect, we should have set expectations with patients early on in the process and tell them that someone will get back to them within one business day – a best practice that has since been incorporated into the Clinpal process.
Another Best Practice resulting from VERKKO is to incorporate a patient-facing call center for 24/7 coverage and also to support multiple languages. A call center can provide a nearly immediate response back to participants and triage any issues with the study sites, removing a significant burden from the study sites.
One aspect of the study that turned out to be critical was the front-loading of compliance management and empowering study participants through the availability of easily digestible information. Instead of relying on the site, the participants themselves were the first line of defense. This had an impact on the overall compliance result, but also in site efficiency as they had less issues to address. Participants also clearly valued having this information available and it improved their engagement with the study. In general, it is much more efficient to avoid compliance issues in the first place, rather than trying to fix them downstream. This best practice has a documented improvement in patient compliance and time-to-completion, as well as, a 200% improvement in site efficiency over the comparative study. In the VERKKO study, it was the patient engagement tools enabled through Clinpal that made the significant difference.
In conclusion, operational success in clinical trial conduct can be significantly improved by deploying the right kind of patient-centric tools to enable patients to self-manage their compliance and to facilitate their convenient participation in trials … And the best news is that these tools are not restricted to only virtual trials, they are readily available to be deployed into nearly every trial.

