The Clinpal Vision
Clinpal is the first end-to-end clinical research platform purpose-built for virtual, hybrid and direct-to-patient studies. Patients log in from anywhere, on any device. Study teams leverage powerful data and analytics across the entire lifespan of the trial. And sites get all functionality all in one system, reducing burden. Clinpal was acquired by Cambridge Cognition in October 2022.
The Clinpal Modules
Why Clinpal?

![]()
Clinpal’s Single Platform – provides end-to-end clinical trial support from patient recruitment and data capture through long-term follow up, with data available on demand
![]()
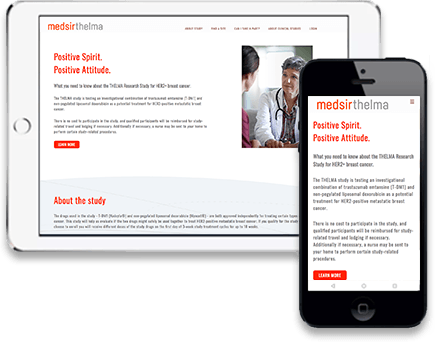
Clinpal’s Innovative Technology – we designed Clinpal to work in multiple formats, with a single online configuration. Configure one time, and Clinpal works for browser, mobile or app users
![]()
Clinpal Process – seamlessly streamlines the clinical trial lifecycle process for all stakeholders, scalable from 1 to 50,000 patients.
![]()
Clinpal’s Patient-friendly Design – An always on dashboard for patients supports them for the life of a study, with eConsent, study information and activity management, reminders, Diary, ePRO and more
Clinpal Solutions
Clinpal Deployments
Clinpal Clinical Trial Experience
| CLIENT | INDICATION | PHASE | DESCRIPTION | COUNTRIES |
|---|---|---|---|---|
| TOP 10 PHARMA | Diabetes | IV | Fully remote, online trial, using a SMART glucose meter integrated with Clinpal to capture blood glucose measurements in real time. 50 patients, 1 study site. Study completed with excellent results. Click here to request more information. | FI |
| TOP 10 PHARMA | Thrombosis | N/A | Bring Your Own Device (BYOD) eCOA validation study. Scientific work to develop a methodology and validate an ePRO patient questionnaire for use with patient's own devices. Involving 25 patients in Spain and Finland. | ES, FI |
| TOP 10 PHARMA | Asthma | III | Site engagement portal with e-Learning and site documents. Deployed to 140 sites, covering 10 e-Learning modules and an extensive document library with over 15 folders. | RU, BG, ZA, NZ, UA, BR, AU, SK, GB, PL, RO, US, CA, GE |
| TOP 10 PHARMA | Asthma | III | Moderate to severe asthma and other related indications. Clinpal is utitlized across this important program as the patient recruitment and engagement solution as well as a site portal. Several studies, 2000+ patients, 400+ sites in 8 countries. | US, CA, MX, JP, AU, ZA, GB, UA |













